Email: info@karssenmetal.com Tel: +86 18147353336
With the continuous development of science and technology, graphite is widely used in semiconductor, photovoltaic power generation, environmental protection, chemical industry, military, aerospace and other fields due to its unique structural characteristics, electrical conductivity, thermal conductivity, lubricity, high temperature resistance and chemical stability. As a necessary non-metallic material for the development of modern industry and high-tech, it plays an increasingly important role in economic development.
Natural graphite can be divided into crystalline graphite and invisible graphite according to its crystallinity. The carbon content of crystalline graphite ore in nature generally does not exceed 10%, the invisible graphite content is high, and the fixed carbon content is generally 60%-80%, up to 95%, but the ore washability is poor.
The impurities contained in graphite are mainly silicate minerals such as potassium, sodium, magnesium, calcium, aluminum, etc. The purification process of graphite is to take effective means to remove these impurities. At present, the methods for purifying graphite mainly include flotation method, alkaline acid method, hydrofluoric acid method, chlorination roasting method, high temperature method, etc.
Flotation Method
Since the surface of graphite is not easily wetted by water, it has good floatability and is easy to separate from impurity minerals. The flotation of raw graphite ore generally uses the positive flotation method first, and then the reverse flotation of the positive flotation concentrate is performed. A high-grade graphite concentrate can be obtained by flotation. The grade of flotation graphite concentrate usually reaches 80% to 90%, and the purity can reach about 98% by multi-stage grinding.
The grade of graphite concentrate purified by flotation method can only reach a certain range, because some impurities are impregnated in the graphite flakes in the form of extremely fine particles. This part of impurities is generally only used as the first step of graphite purification, and the method for further purification of graphite is usually chemical method or high temperature method.

Alkaline method
The principle of purifying graphite by alkali-acid method is to mix NaOH and graphite in a certain proportion and calcine evenly. At a high temperature of 500-700 °C, impurities in graphite such as silicate, aluminosilicate, quartz and other components are mixed with sodium hydroxide. A chemical reaction occurs to generate soluble sodium silicate or acid-soluble sodium aluminosilicate, which is then removed by washing with water to achieve the purpose of desiliconization; Another part of impurities, such as metal oxides, remain in graphite after alkali fusion. In the process, the desiliconized product is leached with acid, so that the metal oxides in it are converted into soluble metal compounds, while impurities such as carbonates in graphite and acid-soluble compounds formed during the alkali leaching process react with acid and enter the liquid phase, and then separated from graphite by filtration and washing. Graphite is chemically inert and stable. It is insoluble in organic solvents and inorganic solvents, and does not react with lye. Except for strong oxidizing acids such as nitric acid and concentrated sulfuric acid, it does not react with many acids, especially resistant to hydrofluoric acid; It does not react with water and water vapor below 6000°C. Therefore, the properties of graphite remain unchanged during the purification process.

Hydrofluoric acid method
When purifying by hydrofluoric acid method, graphite and a certain proportion of hydrofluoric acid are preheated and added to the reactor with agitator. After being fully wetted, the stirring is timed. The temperature of the reactor is controlled by a thermostat. After reaching the specified time the excess acid is removed in time, the filtrate is recycled, the filter cake is washed with hot water to neutrality, dehydrated and dried to obtain the product.
The hydrofluoric acid method is a relatively good purification scheme. It has been industrialized in the 1990s and is commonly used in Europe and the United States. Patents in Japan, France and other countries have introduced that the use of ammonium bifluoride or ammonium fluoride to react with graphite powder with a carbon content of 93% can increase the fixed carbon content of graphite to 99.95%. In view of the huge toxicity of hydrofluoric acid, the production process must have strict safety protection and wastewater treatment system.

Chlorine Roasting
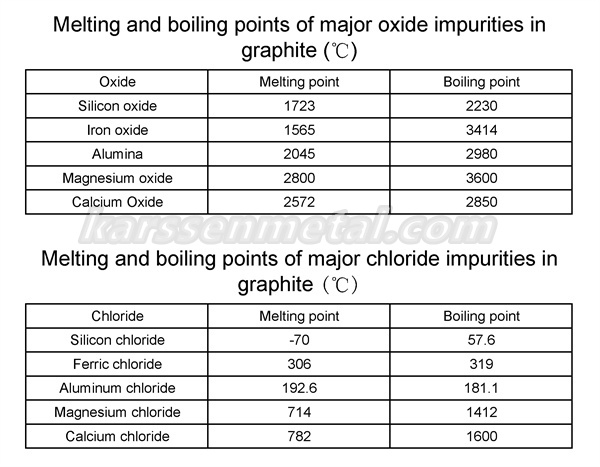
The chlorination roasting method is to add a certain amount of reducing agent to graphite powder, roast it at a certain temperature and a specific atmosphere, and then introduce chlorine gas for chemical reaction, so that the valuable metal in the material is transformed into a gas phase or condensed phase with a lower melting point. The chlorides and complexes escaped, so as to be separated from the remaining components to achieve the purpose of purifying graphite.
The impurities in graphite are heated at high temperature and can be decomposed into simple oxides such as SiO2, Al2O3, Fe2O3, CaO, MgO, etc. under the action of reducing agents. The melting and boiling points of these oxides are high, see Table 1, while And their chlorides or metal complexes formed with other trivalent metal chlorides (such as CaFeCl4, NaAlCl4, KMgCl3, etc.) have lower melting and boiling points, and the vaporization of these chlorides escapes, which improves the purity of graphite. The metal complexes discharged in the gaseous state quickly become condensed phase due to temperature reduction, and the treatment of escaping exhaust gas can be carried out by utilizing this characteristic.
The chlorination roasting method has the advantages of energy saving, high purification efficiency (>98%) and high recovery rate. The toxicity, severe corrosiveness and serious environmental pollution of chlorine gas limit the popularization and application of the chlorination roasting process to a certain extent. Of course, this process is difficult to produce graphite of extreme purity, and the process system is not stable enough, which also affects the application of the chlorination method in actual production. This method needs to be further improved.

High temperature purification method
Graphite is one of the substances with the highest melting and boiling point in nature, with a melting point of 3850±50°C and a boiling point of 4500°C, while the boiling point of silicate minerals is below 2750°C (quartz boiling point), and the boiling point of graphite is much higher than the impurities like silicate contained in it. This characteristic is the theoretical basis for the high temperature purification of graphite.
Put the graphite powder directly into the graphite crucible, and heat it to 2300-3000℃ in a purification furnace with inert gas and freon protective gas, and keep it for a period of time, the impurities in the graphite will overflow, so as to realize the purification of the graphite. The high-temperature method generally uses high-carbon graphite with a carbon content of more than 99% purified by flotation or chemical method as the raw material, and the graphite can be purified to 99.99%. If the process conditions are further improved and the quality of the crucible is improved, the purity can reach 99.995% or above.
The high-temperature method can produce more than 99.99% ultra-high-purity graphite, but the fixed carbon of the raw material is required to be more than 99%, and the equipment is expensive, the investment is huge, and the production scale is limited. The electric furnace heating technology is strictly required, and it is necessary to isolate the air, otherwise the graphite will start to be oxidized when the temperature rises to 450 ℃ in the hot air. The higher the temperature, the greater the loss of graphite. Only special industries (such as national defense, aerospace, etc.) that require very high graphite quality use the high-temperature method to produce high-purity graphite in small batches.

Several methods of graphite purification have their own advantages and disadvantages, and they all have certain defects. The alkaline-acid method is easy to operate, has low production cost, and has lower requirements for production conditions, but the content of graphite fixed carbon produced is low, which cannot reach 99.9% from the current point of view. The hydrofluoric acid method has good impurity removal effect and high fixed carbon content in the product, but hydrofluoric acid is highly toxic and corrosive, and requires strict safety protection measures and production conditions, and the waste water is not easy to treat. The chlorination roasting method also requires strict sealing due to the toxicity and strong corrosiveness of chlorine gas. The high-temperature method can produce very high-grade high-purity graphite, which can be used in high-tech fields such as aerospace and semiconductors. However, it is necessary to specially design and build a high-temperature furnace, which has high cost, high energy consumption and relatively harsh production conditions.
In the smelting process of non-ferrous metals su
Isostatic graphite blocks are an important graph
Graphite rotor belongs to graphite material, whi
Contact: Bateer
Phone: +86 18147353336
Tel: +86 18147353336
Email: info@karssenmetal.com
Add: Room D204-2203, Innovation Building, Baotou Light Industry Vocational Technical College, 19 Jianhua Road, Qingshan District, Baotou City, Inner Mongolia, China.