Email: info@karssenmetal.com Tel: +86 18147353336
Carbon material VS graphite material
Carbon is an element name, and carbon materials are actually carbon materials containing more amorphous carbon structures. The graphite material is a carbon material mainly composed of graphite crystals. Both have many similarities, but there are significant differences.
Because carbon materials and graphite materials have many similarities in appearance and characteristics, in reality, many people do not know how to distinguish the relationship between the two. This article will give a detailed introduction to this.
The three most common allotropes of carbon exist: amorphous carbon, graphite, and diamond.
Diamond
Diamond is a mineral composed of carbon. Diamond is the hardest substance in nature, known as "the king of hardness" and the king of gems. The angle of the crystals of diamond is 54 degrees 44 minutes 8 seconds. In the 1950s, the United States used graphite as raw material to successfully manufacture synthetic diamond under high temperature and pressure. So far, synthetic diamond has been widely used in production and life.
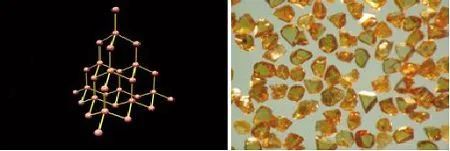
Diamond molecular structure and appearance
Amorphous carbon
Amorphous carbon generally refers to charcoal, coke, bone char, sugar charcoal, activated carbon and carbon black. Except for the carbon content of bone char, which is about 10%, the other main components are elemental carbon. Coal is naturally occurring amorphous carbon that contains some compounds consisting of carbon, hydrogen, nitrogen, etc.
The so-called amorphous carbon does not refer to the shape in which these substances exist, but to its internal structure. In fact, their internal structure is not a real amorphous body, but a crystal with a structure similar to that of graphite, but the layered structure formed by the hexagonal ring plane of carbon atoms is disordered and irregular, the crystal formation is defective, and the crystal particles are tiny and contain a small amount of impurities.
The structure of most of the amorphous carbon is that the molecular fragments of the graphite layer structure are roughly parallel to each other and are randomly stacked together, which can be referred to as a turbostratic structure. The layers or fragments are connected by carbon atoms in the form of tetrahedral bonds of diamond structure. When the proportion of carbon atoms in this tetrahedron is large, the amorphous carbon will be relatively hard, such as coke and glassy carbon.
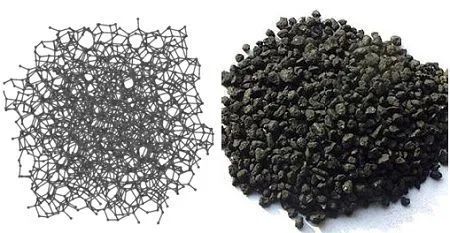
Amorphous carbon molecular structure and appearance diagram
Graphite
Graphite is an allotrope of elemental carbon. Each carbon atom is surrounded by three other carbon atoms (multiple hexagons arranged in a honeycomb pattern) to form a covalent molecule. Because each carbon atom emits an electron, and those electrons are free to move, graphite is an electrical conductor.
Graphite is divided into natural graphite and artificial graphite. Natural graphite ore is further divided into earthy graphite and flake graphite, of which flake graphite is a better natural graphite.
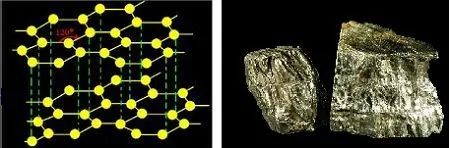
Graphite molecular structure and appearance
The similarities between carbon materials and graphite materials are that they are both non-metallic solid materials dominated by carbon elements; the difference is that carbon materials are materials composed of non-graphitic carbon, while graphite materials are composed of graphitic carbon. Since the difference between carbon materials and graphite materials is not big in nature, many carbon products are also named "graphite XX". This is also the source of confusion between the two lexical concepts of graphite and carbon. For example, the large electrode commonly used in the steelmaking industry is called "graphite electrode". In fact, it is a carbon product, not an electrode made of graphite in the true sense. In terms of appearance, common carbon products are large in size, such as metallurgical electrodes, lining bricks (carbon blocks) of smelting furnaces, carbon radiators and so on. Graphite materials, like carbon materials, can be made into industrial products such as electrodes and refractory materials. But because of its more excellent characteristics, it has applications in many high-end fields.
Isostatic graphite blocks are an important graph
Graphite rotor belongs to graphite material, whi
Graphite sheets have many important roles in the
Contact: Bateer
Phone: +86 18147353336
Tel: +86 18147353336
Email: info@karssenmetal.com
Add: Room D204-2203, Innovation Building, Baotou Light Industry Vocational Technical College, 19 Jianhua Road, Qingshan District, Baotou City, Inner Mongolia, China.